SELENIUM BINDING PROTEIN (SBP)
Selenium Βinding Protein (SBP, originally termed SBP56) was identified in mouse liver as a cytosolic protein that could bind radioactive selenium. SBPs are highly conserved proteins present in a wide array of species across all kingdoms and are likely to be involved in selenium metabolism. In Arabidopsis, the selenium binding protein (SBP) gene family comprises three genes (AtSBP1, AtSBP2 and AtSBP3). AtSBP1 and AtSBP2 are clustered in a head-to-tail arrangement on chromosome IV, while AtSBP3 is located on chromosome III. All three SBP genes are upregulated in response to externally applied selenium compounds and the antioxidant NAC selectively downregulates SBP2. Although the effect on SBP2 levels was the most prominent, in all cases, the concurrent exposure of plants to selenite and the antioxidant supressed the expression of the SBP genes. We have shown that (at least) SBP1 expression is tightly linked to detoxification processes related to oxidative stress, since it is downregulated in the presence of NAC in selenium-treated plants. Furthermore, our results suggest that SBP genes may participate in the mechanisms that sense redox imbalance.
Selenium Βinding Protein (SBP, originally termed SBP56) was identified in mouse liver as a cytosolic protein that could bind radioactive selenium. SBPs are highly conserved proteins present in a wide array of species across all kingdoms and are likely to be involved in selenium metabolism. In Arabidopsis, the selenium binding protein (SBP) gene family comprises three genes (AtSBP1, AtSBP2 and AtSBP3). AtSBP1 and AtSBP2 are clustered in a head-to-tail arrangement on chromosome IV, while AtSBP3 is located on chromosome III. All three SBP genes are upregulated in response to externally applied selenium compounds and the antioxidant NAC selectively downregulates SBP2. Although the effect on SBP2 levels was the most prominent, in all cases, the concurrent exposure of plants to selenite and the antioxidant supressed the expression of the SBP genes. We have shown that (at least) SBP1 expression is tightly linked to detoxification processes related to oxidative stress, since it is downregulated in the presence of NAC in selenium-treated plants. Furthermore, our results suggest that SBP genes may participate in the mechanisms that sense redox imbalance.
SBP INTERACTING PROTEINS
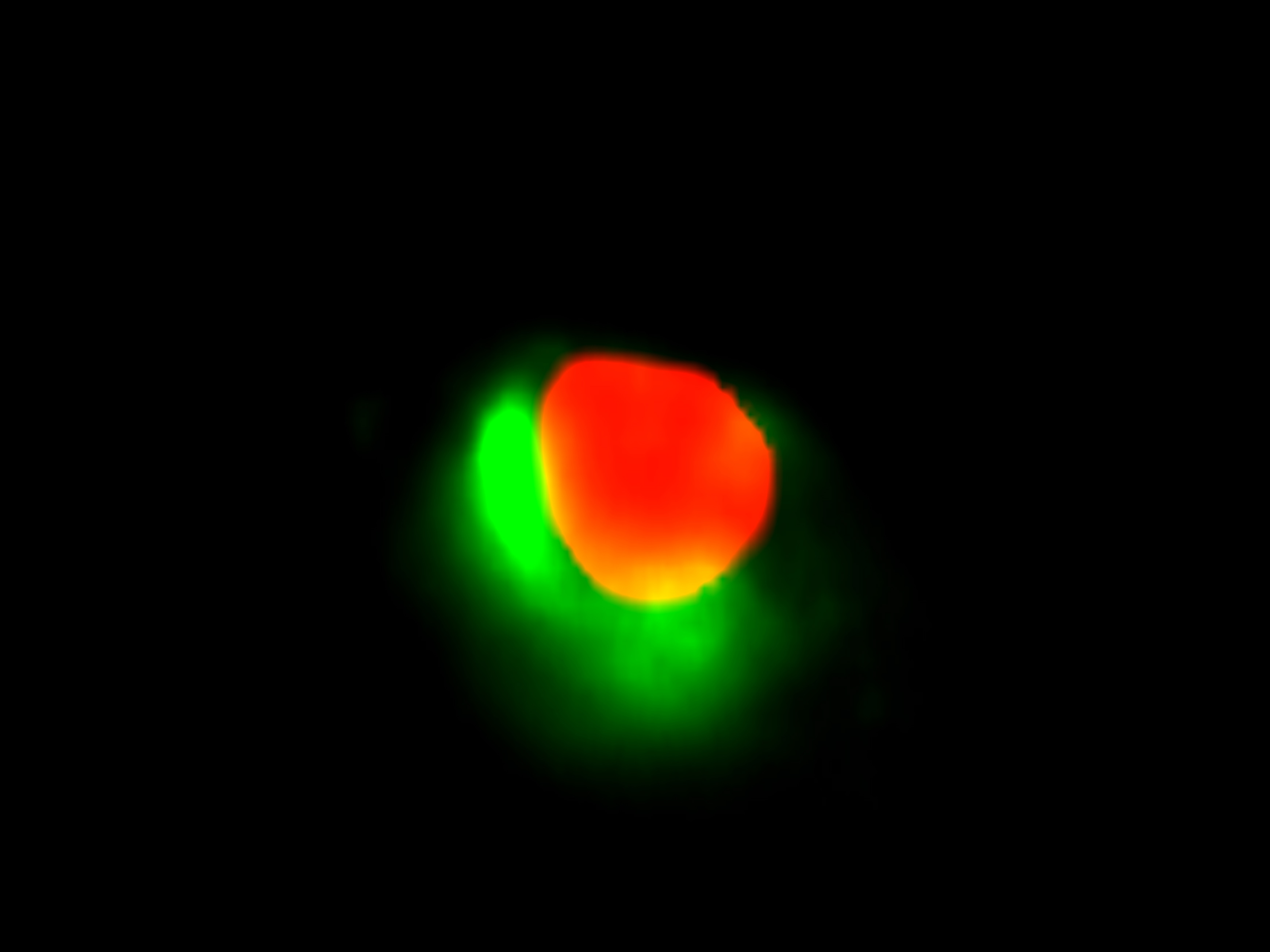
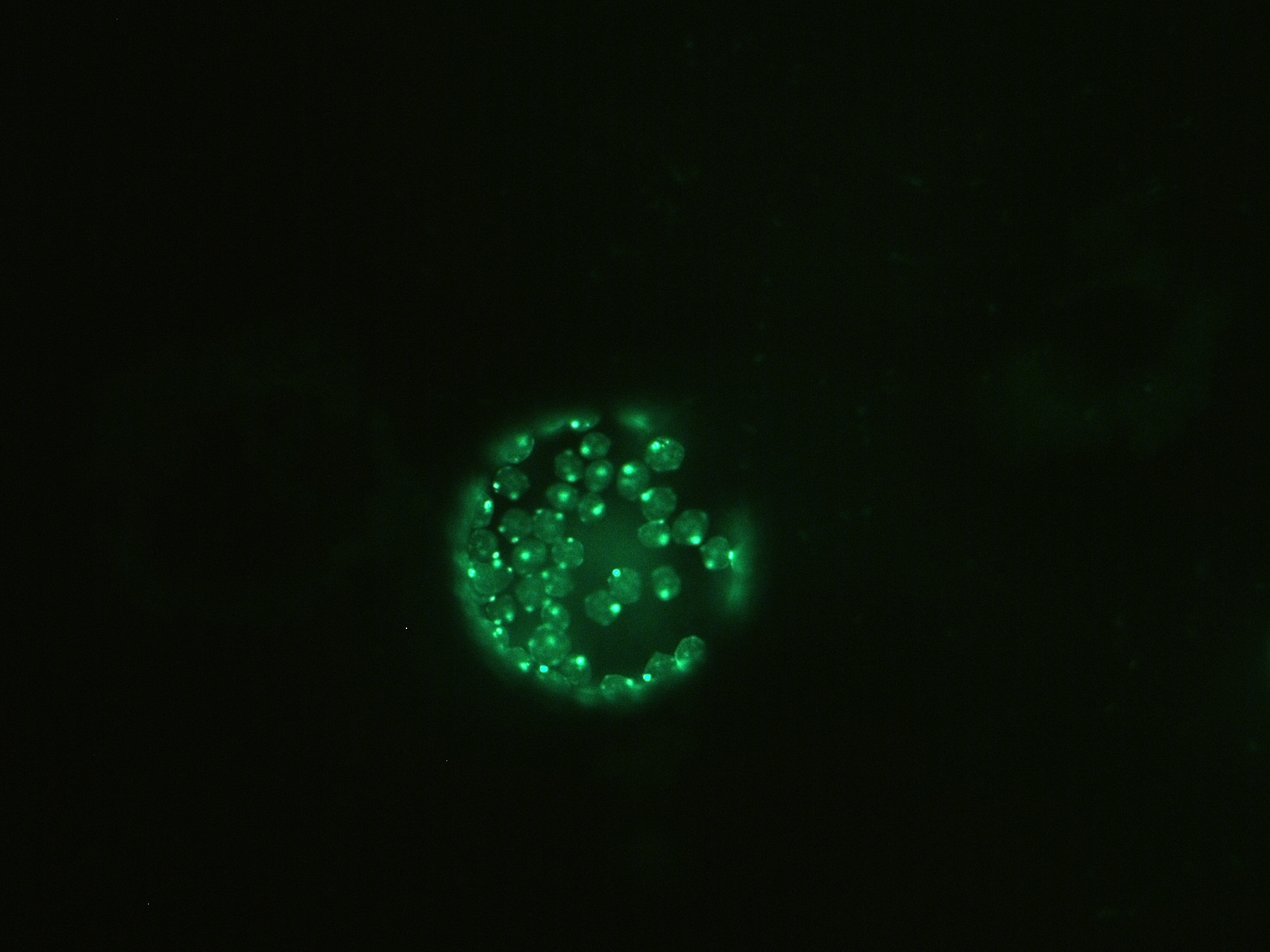
In vitro binding assays have shown that in Arabidopsis SBP1 participates in a novel protein network consisting of at least SBP, a NADP-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a fructose-bisphosphate aldolase (FBA), that is possibly part of the physiological regulation and metabolism of selenium (Agalouet al., 2006) given the fact that in Escherichia coli GAPDH and a prokaryotic aldolase (deoxyribose-5-phosphate aldolase; DPA) were shown to bind selenium (Lacourciere et al., 2002). A major step towards understanding SBP1 function in plants and its involvement in Se metabolism and detoxification mechanisms was the identification of the selenium binding site and the involvement of two Cys residues in AtSBP1, as well as the function of selenite (SeO32-) reduction that this protein was shown to drive (Schildet al., 2014). To further characterize the protein network that AtSBP1 protein participates in, we studied its interaction with AtGRXS14. Furthermore, we have showed that SBP protein family interacts with AtGRXS16 that also contains a PICOT domain, as AtGRXS14.
In vitro binding assays have shown that in Arabidopsis SBP1 participates in a novel protein network consisting of at least SBP, a NADP-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a fructose-bisphosphate aldolase (FBA), that is possibly part of the physiological regulation and metabolism of selenium (Agalouet al., 2006) given the fact that in Escherichia coli GAPDH and a prokaryotic aldolase (deoxyribose-5-phosphate aldolase; DPA) were shown to bind selenium (Lacourciere et al., 2002). A major step towards understanding SBP1 function in plants and its involvement in Se metabolism and detoxification mechanisms was the identification of the selenium binding site and the involvement of two Cys residues in AtSBP1, as well as the function of selenite (SeO32-) reduction that this protein was shown to drive (Schildet al., 2014). To further characterize the protein network that AtSBP1 protein participates in, we studied its interaction with AtGRXS14. Furthermore, we have showed that SBP protein family interacts with AtGRXS16 that also contains a PICOT domain, as AtGRXS14.
enod40 RNA
Plant long noncoding RNA enod40 is involved in the regulation of symbiotic associations with bacteria. The presence of enod40 genes in plants that do not form such symbioses indicates its other roles in cell physiology. Enod40 RNAs form several structured domains, conserved to different extents. Due to relatively low sequence similarity, identification of enod40 sequences in plant genomes is not straightforward, and many enod40 genes remain unannotated even in complete genomes. Comparative structure analysis and sequence similarity searches located enod40 genes and determined enod40 RNA structures in nitrogen-fixing clade plants and in grasses. The structures combine conserved features with considerable diversity of structural elements, including insertions of structured domain modules originating from transposable elements. Remarkably, these insertions contain sequences similar to tandem repeats and several stem-loops are homologous to microRNA precursors.